Biomedical Materials Translational Facility
Biomedical Materials Translational Facility (BMTF)
The SIEF funded Biomedical Materials Translation Facility (BMTF) is located at Clayton in the Monash Technology Precinct and central to the Monash Innovation Cluster.
The BMTF research infrastructure initiative is a partnership between Monash University and CSIRO with the aim of helping industry translate discoveries into market ready medical technologies.
This initiative provides expertise and capabilities in three synergistic areas critical to the translation of biomedical materials research:
- Clean room materials synthesis, fabrication and surface coating.
- High throughput biological testing and evaluation of materials.
- Large animal models and real-time non-invasive imaging for preclinical studies.
The development of new and improved medical technologies, particularly medical devices, faces technical and regulatory challenges, and invariably requires a multidisciplinary approach with research expertise drawn from a range of disciplines such as materials science, surface chemistry, biomedical engineering, cellular biology as well as pre-clinical and ultimately clinical assessment. In the context of the BMTF, these capabilities provide an environment in which the existing research expertise from partners and collaborators will be enhanced to enable the development of new materials for devices, diagnostics, delivery technologies, imaging agents and the assessment of these materials through new, non-invasive, in situ imaging techniques. They also allow discoveries to be accelerated through the subsequent development phases of scale-up, prototype evaluation and biological testing. Importantly and uniquely, this research infrastructure capabilities will be housed within facilities operating under appropriate quality management systems. The Monash and CSIRO facilities are currently operating under ISO 9001:2015 This will enable new materials, devices and processes to be developed within the framework needed for subsequent in vivo evaluation and adoption by industry partners.
The Monash Technology Precinct will be a focal point to draw in, engage and stimulate industry. Within Australia’s medical technologies sector there are a range of highly innovative companies with high growth potential. With a focus on translational biomedical materials research, companies will be provided with a means to proactively engage earlier in the R&D process.
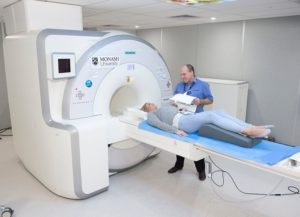
The MRI-PET scanner is part of the Biomedical Material Translation Facility at Clayton, established with funding from SIEF.
The MRI-PET Scanner at Monash Biomedical Imaging is the only research dedicated scanner in Victoria. (Image: Monash University)
Capabilities include the following infrastructure and expertise:
- PET MRI Scanner housed at Monash University, for high quality non-invasive imaging,
- Tissue Bioprinter at Monash University, for 3D printed tissue design and to improve accuracy of pre-clinical and clinical analysis,
- ISO7 Clean room at CSIRO, for housing state-of-the-art equipment for synthesis, fabrication, processing and surface modification of components for new biomedical devices including implants,
- Synthesis, fabrication and surface coating equipment housed in the clean room at CSIRO, for the synthesis, material processing and fabrication of components of biomedical devices and implants for preclinical trials and industry evaluation,
- High throughput biological testing equipment housed in a physical containment level 2 (PC2) laboratory at CSIRO for rapid in vitro biological assessment of new materials and coatings.
These capabilities are integrated within the greater Monash Technology Research Platforms and CSIRO research infrastructure allowing projects to access other core capabilities across these organisations and the Monash Technology Precinct.
Three years of operations has produced many research and industry outcomes. Research and industry projects are progressing through all capabilities and the facilities are working together to ensure seamless processes between all capabilities and organisations.

CSIRO Biomedical Materials Translation Facility (Image: Monash University)
The investment in the advanced medtech capabilities by SIEF, CSIRO and Monash University has been leveraged by significant increases in industry usage and successful collaborative activities including:
- CRC-P Grant ($2.18M) involving Latrobe Uni and biomedical industry partners.
- Symposium on Regulatory Fundamentals in Medical Devices – this symposium was organised under the BMTF banner, strongly supported by the TGA and attracted more than one hundred attendees.
- Joint Industry/Monash successful project funded to use MR-PET by MTPConnect Biomedical translation Bridge program worth $1M.
- MBI successful in NCRIS National Imaging Facility funding to expand the radiopharmaceutical precision capabilities – providing significant expansion of capabilities for the MR-PET and radiopharmaceutical capabilities.
- Monash University/QUT collaboration in leading the ARC Training Centre in Cell and Tissue Engineering Technologies with twenty six industry partners.
- More than ten joint Industry/CSIRO collaborative projects carried out in the CSIRO clean room and PC2 laboratories.
